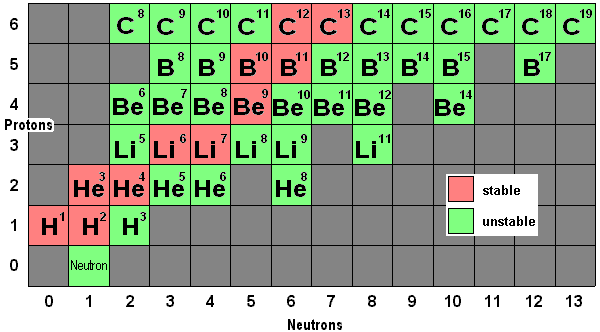
Isotopes of the same element have similar chemical properties but different physical properties.
Since Chlorine-35 and Chlorine-37 have the same number of proton and electron but different number of neutron, they are called isotopes of chlorine. Thus, the average nucleon number of chlorine will be [(75/100) x35 ] + [(25/100) x37] = 35.5
| Chlorine Atoms | No. of Proton | No. of Electron | No. of Neutron |
| Chlorine-35 | 17 | 17 | 18 |
| Chlorine-37 | 17 | 17 | 20 |

http://alexteoh.com/Atomic%20Structure_files/image003.jpg
http://www.wordwizz.com/images/isotopes.gif
No comments:
Post a Comment