
http://www.micromountain.com/sci_diagrams/at_struct/at_struct_assets/sulphur_lab_usa.jpg
 Extra^^
Extra^^http://www.webelements.com/_media/elements/kossel_diagrams/S.jpg

http://chemwiki.ucdavis.edu/@api/deki/files/4996/=Sulfate%20pt.%202.jpg


http://0.0.0.3/4.bp.blogspot.com/-Yy6H9WZQsK0/ThFsS_xMXJI/AAAAAAAAAEQ/bB0e3S_VoqU/s1600/Untitled-4jpg.jpg
Ionic bonds are formed when metallic atoms has given away their valence electrons to the non-metallic atoms.
The transfer of valence electrons from the metallic to the non-metallic atoms enables both atoms to achieve stablility. (stable noble gas configuration)
oppositely charges ions are formed which will attacted to each other due to the strong electrostatic forces of attraction, that form the ionic bonds.
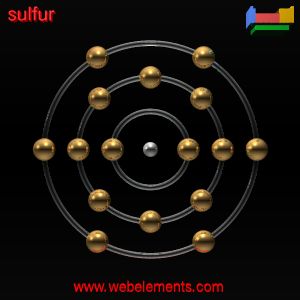
THerefore, i drew the sulfur atom that way as its has 16 electrons .
Its electronic configuration is (2.8.6). It need 2 extra valence electrons to become stable . Thus,sulfide ion is drawn that way because it has gained 2 valence electron . Hence, its electronic configuration has become ( 2.8.8 ) after recieving two electron. and is now negatively charge
No comments:
Post a Comment