Kaiqi-
She explained her works clearly, it is detailed and easy to understand. Her diagrams are clear and that helps me to understand a lot. i like her answers^^
Sunday, July 17, 2011
Comment 2
Aleen :
Her Answer are detailed. However, the words are too small and its difficult for mii to read. She should also separate her answers into paragraphs. And that would be better.. greatly done:))
Comment 1
Ysabelle-
Her answer is interesting because it is filled with different colour that make readers feel like reading it till the end. It can be seen that she does puts in the effort to make her answers well explained. The blog is also simple and nice. Neatly done^^
Her answer is interesting because it is filled with different colour that make readers feel like reading it till the end. It can be seen that she does puts in the effort to make her answers well explained. The blog is also simple and nice. Neatly done^^
Sunday, July 10, 2011
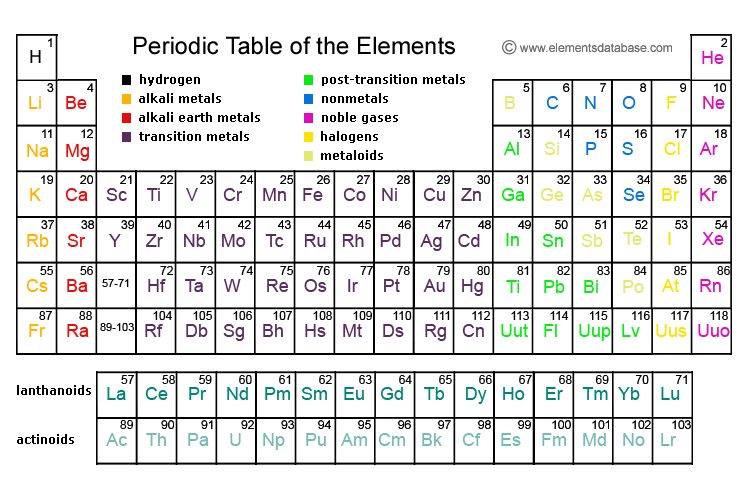
5. Sodium is a metal and sulfur is a non-metal....why we classify them this way??
http://www.elementsdatabase.com/Images/periodic_table.gif

http://www.chemglobe.org/ptoe/periodic.png
Sulfur is a non-metal because:
1. it does not conduct electricity
2. it will gain electrons to complete the valence shell
3. it lies on top of the stair case on the table
Sodium is a metal because:
1. it conducts electricity
2. it will lose electron to complete the valence shell
3. it lies below the staircase (group1 )
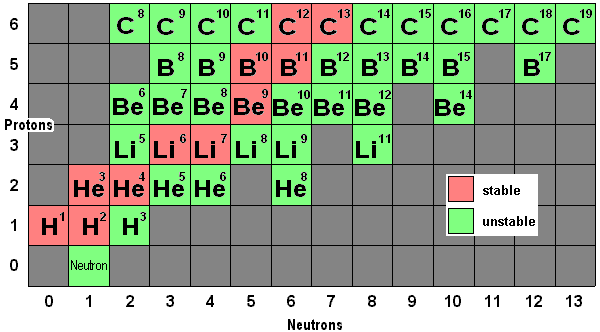
4. Chlorine-35 atom and Chlorine-37 atom are called isotopes...Use these two examples to explain what is 'isotopes'.
Isotopes are atoms of the same element that have different nucleon number (mass no.) or different number of neutrons.
Isotopes of the same element have similar chemical properties but different physical properties.
Since Chlorine-35 and Chlorine-37 have the same number of proton and electron but different number of neutron, they are called isotopes of chlorine. Thus, the average nucleon number of chlorine will be [(75/100) x35 ] + [(25/100) x37] = 35.5

http://alexteoh.com/Atomic%20Structure_files/image003.jpg
http://www.wordwizz.com/images/isotopes.gif
Isotopes of the same element have similar chemical properties but different physical properties.
Since Chlorine-35 and Chlorine-37 have the same number of proton and electron but different number of neutron, they are called isotopes of chlorine. Thus, the average nucleon number of chlorine will be [(75/100) x35 ] + [(25/100) x37] = 35.5
| Chlorine Atoms | No. of Proton | No. of Electron | No. of Neutron |
| Chlorine-35 | 17 | 17 | 18 |
| Chlorine-37 | 17 | 17 | 20 |

http://alexteoh.com/Atomic%20Structure_files/image003.jpg

http://www.wordwizz.com/images/isotopes.gif
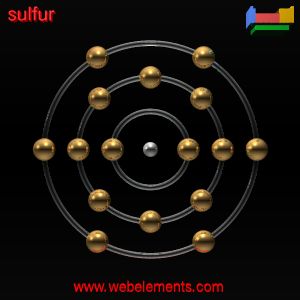
3. Draw the atomic structure of a sulfur atom and a sulfide ion....explain why you draw it this way.

http://www.micromountain.com/sci_diagrams/at_struct/at_struct_assets/sulphur_lab_usa.jpg
 Extra^^
Extra^^http://www.webelements.com/_media/elements/kossel_diagrams/S.jpg

http://chemwiki.ucdavis.edu/@api/deki/files/4996/=Sulfate%20pt.%202.jpg


http://0.0.0.3/4.bp.blogspot.com/-Yy6H9WZQsK0/ThFsS_xMXJI/AAAAAAAAAEQ/bB0e3S_VoqU/s1600/Untitled-4jpg.jpg
Ionic bonds are formed when metallic atoms has given away their valence electrons to the non-metallic atoms.
The transfer of valence electrons from the metallic to the non-metallic atoms enables both atoms to achieve stablility. (stable noble gas configuration)
oppositely charges ions are formed which will attacted to each other due to the strong electrostatic forces of attraction, that form the ionic bonds.
THerefore, i drew the sulfur atom that way as its has 16 electrons .
Its electronic configuration is (2.8.6). It need 2 extra valence electrons to become stable . Thus,sulfide ion is drawn that way because it has gained 2 valence electron . Hence, its electronic configuration has become ( 2.8.8 ) after recieving two electron. and is now negatively charge
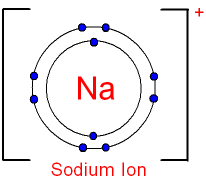
2. Draw the atomic structure of a sodium atom and a sodium ion....explain why you draw it this way.

https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEhlF5vDL1_N-xq7plD6YADEOqZOn_0TwwOtFxNJxPzEmLsDj3I4-_kYVwdvngGZucfOZqdmlKdG5bBE1LQJAApqhVWY4ArXLh5slCDMKGu7b7EGWibg32m_mtGmQnGbWEeSoWQ6OXjxG47c/s320/Sodium-Atom-Sodium-Ion.gif

http://iss.cet.edu/electricity/pages/images/b/b11_5.jpg
Ionic bonds are formed when metallic atoms has given away their valence electrons to the non-metallic atoms.
The transfer of valence electrons from the metallic to the non-metallic atoms enables both atoms to achieve stablility. (stable noble gas configuration)
oppositely charges ions are formed which will attacted to each other due to the strong electrostatic forces of attraction, that form the ionic bonds.
THerefore, i drew the sodium atom that way as its has 11 electrons .

http://www.google.com.sg/imgres?imgurl=http://www.scioly.org/w/images/1/1b/Electron_shell_011_Sodium.svg.png&imgrefurl=http://www.scioly.org/wiki/GMOA_Notes&usg=__BWr41JgSP1sAjIKSHoywbKX933Q=&h=194&w=180&sz=10&hl=en&start=14&zoom=1&itbs=1&tbnid=M0tsb3vKAyYmyM:&tbnh=103&tbnw=96&prev=/images%3Fq%3Dthe%2Batomic%2Bstructure%2Bof%2Ba%2Bsodium%2Batom%2Band%2Ba%2Bsodium%2Bion.%26hl%3Den%26gbv%3D2%26biw%3D1003%26bih%3D622%26tbm%3Disch&ei=w7MZTqOSLsXVrQfGzPzPAQ
Its electronic configuration is (2.8.1). It has 1 extra valence electron. thus, sodium ion is drawn that way because it has given away it in order to become stable . Hence, its electronic configuration has become ( 2.8 ) after that removal of that electron. and is now positely charge
http://www.gcsescience.com/sodium-ion.gif
1. What does an atom looks like? What are the sub-atomic particles inside it.....(talk about electrons, neutrons, protons, electron shells, nucleus....)
Atoms are made up of three basic particles (sub-atomic ): protons, neutrons and electrons.
The properties of the three particles are shown in the table below:
The structure of an atom:


THe composition of protons, neutrons and electrons in an atom can be determined from its proton number ( atomic number)and nucleon number (mass number).
The proton number is defined as the number of protons in an atom.
The nucleon number is defined as the total number of protons and neutrons in the atom.
Since an atom is electronically neutal, number of protons should be equal to number of electrons in the atoms.
An atom cann be written with a symbol. Example:

MEANING:
1. The atom is sodium
2.Has 11 protons and electrons
3.Nucleon(mass)number of 23 (neutrons+ protons)
4. Has(23-11=) 12 neutrons
The way in which electrons are arranged in an atom among the various energy shells is called its electron configuration.
Each shell cann only hold up to a certain number of electrons
And each shell must be completely filled before going to the next level.
Examples:


Note that:
Protons and Neutrons are found in the nucleus
Electrons are found in the electron shell.
An electron is negatively charged while a proton is positively charged.
http://www.google.com.sg/imgres?imgurl=http://www.scioly.org/w/images/1/1b/Electron_shell_011_Sodium.svg.png&imgrefurl=http://www.scioly.org/wiki/GMOA_Notes&usg=__BWr41JgSP1sAjIKSHoywbKX933Q=&h=194&w=180&sz=10&hl=en&start=14&zoom=1&itbs=1&tbnid=M0tsb3vKAyYmyM:&tbnh=103&tbnw=96&prev=/images%3Fq%3Dthe%2Batomic%2Bstructure%2Bof%2Ba%2Bsodium%2Batom%2Band%2Ba%2Bsodium%2Bion.%26hl%3Den%26gbv%3D2%26biw%3D1003%26bih%3D622%26tbm%3Disch&ei=w7MZTqOSLsXVrQfGzPzPAQ
The properties of the three particles are shown in the table below:
| Sub-atomic Particles | Relative Mass |
| Electron | 1 / 1840 |
| Proton | 1 |
| Neutron | 1 |
The structure of an atom:


THe composition of protons, neutrons and electrons in an atom can be determined from its proton number ( atomic number)and nucleon number (mass number).
The proton number is defined as the number of protons in an atom.
The nucleon number is defined as the total number of protons and neutrons in the atom.
Since an atom is electronically neutal, number of protons should be equal to number of electrons in the atoms.
An atom cann be written with a symbol. Example:

MEANING:
1. The atom is sodium
2.Has 11 protons and electrons
3.Nucleon(mass)number of 23 (neutrons+ protons)
4. Has(23-11=) 12 neutrons
The way in which electrons are arranged in an atom among the various energy shells is called its electron configuration.
Each shell cann only hold up to a certain number of electrons
And each shell must be completely filled before going to the next level.
Examples:


Note that:
Protons and Neutrons are found in the nucleus
Electrons are found in the electron shell.
An electron is negatively charged while a proton is positively charged.
http://www.google.com.sg/imgres?imgurl=http://www.scioly.org/w/images/1/1b/Electron_shell_011_Sodium.svg.png&imgrefurl=http://www.scioly.org/wiki/GMOA_Notes&usg=__BWr41JgSP1sAjIKSHoywbKX933Q=&h=194&w=180&sz=10&hl=en&start=14&zoom=1&itbs=1&tbnid=M0tsb3vKAyYmyM:&tbnh=103&tbnw=96&prev=/images%3Fq%3Dthe%2Batomic%2Bstructure%2Bof%2Ba%2Bsodium%2Batom%2Band%2Ba%2Bsodium%2Bion.%26hl%3Den%26gbv%3D2%26biw%3D1003%26bih%3D622%26tbm%3Disch&ei=w7MZTqOSLsXVrQfGzPzPAQ
Subscribe to:
Comments (Atom)