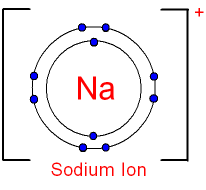
i) Write an ionic equation for the reaction.
ZnCO3 (s) + 2H+ (aq) --> Zn 2+ (aq) + H2O (l) + CO2 (g)
ii) Why excess zinc carbonate is used?
Is to ensure that all the hydrochloric acid is completely reacted, before filtering the excess unreacted zinc carbonate from the zinc chloride solution.
iii) Briefly explain how the zinc chloride crystals can be obtained.
Step 1: Add the excess zinc carbonate to a beaker of hydrochloric acid until there is excess zinc carbonate seen.
Step 2: Filter to remove the excess unreacted zinc carbonate.
Step 3: Evaporate the zinc chloride solution to remove the water and to obtain a saturated salt solution (heat slowly to prevent salt to jump out)
Step 4: Leave the hot saturated solution to cool down. When the hot saturated salt solution cools down, zinc chloride crystals is obtained.



 Extra^^
Extra^^